- Message
- About
- Events & Activities
- Team
- Join Us
- Newsletter
- Informative Videos
- Resources
Welcome Message from the Coordinator Team
“Welcome to Pakistan’s first ever Patient & Public Involvement and Engagement (PPIE) group! We hope you join us in our effort to work together with patients, families, and the public to make our health research more relevant and useful here at Ziauddin University”

Messages from our Collaborators
“Being part of a Patient & Public Involvement and Engagement Group is not only personally fulfilling, but it also plays a crucial role in transforming healthcare for the better. Through active participation, we can drive positive change, amplify the voices of those directly impacted, and create a more patient-centered, equitable, and responsive healthcare system."
Mr. Zahyd Shuja
PPIE Member
“Health research is all about patients, so it makes perfect sense to me that we meaningfully involve patients in all aspects of the clinical trial process. I feel very honoured to get to work with patients as partners and find out from them what diseases we should research and how research should be done.”
Dr. Arishay Hussaini
Co-lead for the PPIE group
About Patient & Public Involvement and Engagement
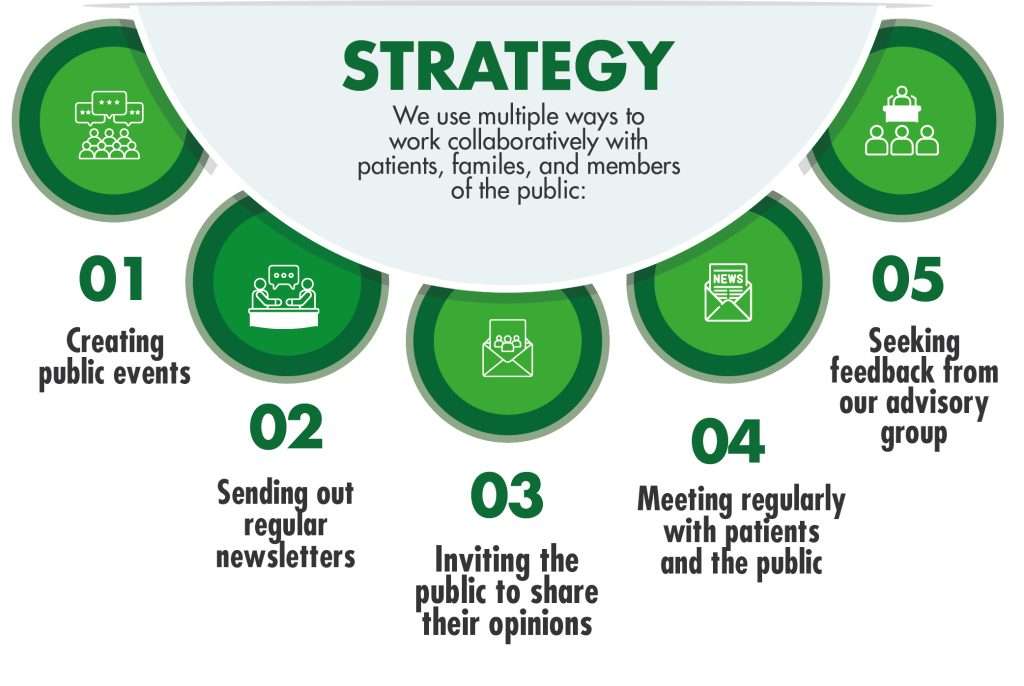
Patient & Public Involvement and Engagement, or PPIE for short, is all about researchers working together with patients, families, and the public to shape and influence research. This includes deciding what conditions and treatments to study, how research methods could be improved, and what changes should be made as a result of research findings. The main goal of PPIE is to ensure that research is acceptable, relevant, and beneficial to patients and the public.
The PPIE approach is internationally-recognized and increasingly used to improve the quality of research in all areas of health. Our group, based at the Clinical Trials Unit at Ziauddin University, is bringing PPIE to Pakistan to improve the way we conduct clinical trials because we strongly believe in the power of working together with patients.
Our involvement and engagement in clinical trials align with internationally accepted standards, and the core six standards we follow have been adapted from the ‘UK Standards for Public Involvement’ created by the National Institute for Care and Health Research (NIHR) and partner organizations in the United Kingdom.

Event: Join us to celebrate International Clinical Trials Day: 20th May
The International Clinical Trials Day on Monday 20th of May marks 250 years since the first ever clinical trial. The Clinical Trials Unit at Ziauddin University is celebrating the Day by opening its doors to patients and the public. Staff and representatives from our patient group will talk about the work they do, what the Clinical Trials Unit has achieved so far and what the future holds. There will be a world premiere of a short video explaining clinical trials created in collaboration with an animation and video team based in Karachi. Free refreshments will be served as the public has the opportunity to tour our Clinical Trials Unit.
Join us for a casual and informative open day to discover how clinical trials connect to you and your community on Monday 20th of May in the Clinical Trials Unit of Ziauddin University Hospital at Clifton Campus (Basement Floor) from 12-2pm and 6-8pm. You can join us at any time! Contact us on 03213660201 or [email protected] if you would
like more information
22 November 2023. Watch one of our PPIE co-coordinators, Dr. Arishay Hussaini’s, presentation at the global PPIE meeting of the REMAP-CAP trial, where she shared the lessons learned in establishing a public engagement group in a low- and middle-income country. The meeting was attended by researchers from around the world, including Australia, Japan, Ireland and Canada, and attendees discussed ways in which to incorporate the voices of patients from low-resource settings more meaningfully in the design and conduct of REMAP-CAP. Our PPIE group at Ziauddin University was congratulated for overcoming challenges and inspiring others by showing that engagement is possible in all sorts of environments.
8 December 2023. Dr. Timo Tolppa (PPIE group co-coordinator) attended a critical care medicine conference in Nepal and shared our success at Ziauddin University in establishing a patient engagement group at the Clinical Trials Unit. Attendees expressed excitement and interest in learning more about patient and public engagement, with some wanting to establish a similar group in Nepal. Clearly, our PPIE group is serving as an example for others in the region, demonstrating that patient engagement is feasible and valuable. As our own work continues, we hope to inspire many others in Pakistan, Nepal and beyond to embark on the rewarding journey of public engagement.
2 February 2024. The Indian Registry of Intensive Care Network invited Dr. Timo Tolppa (PPIE group co-coordinator) to their annual meeting in Chennai to speak about patient and public engagement in research. He shared the lessons we learned from our success at Ziauddin University in starting a patient engagement group at the Clinical Trials Unit. Attendees wanted to know how our group overcame challenges in order to create such a group in a low-resource setting and were inspired to establish their own groups. Our PPIE group has offered our support to create more groups in South Asia, as we believe in strengthening the patient voice in research everywhere.
Event: Research Ethics
11 November 2023. Our PPIE group met at the Center of Biomedical Ethics and Culture (CBEC) at the Sindh Institute of Urology and Transplantation (SIUT) to discuss research ethics. With the addition of our newest member, our “Fellowship of the Willing” was introduced to differences between medical practice and research; trial participants and patients; doctors and researchers. This was the first of two workshops on informed consent processes in clinical trials. As a result of this successful workshop, the group is very excited to bring their voice to the consent processes at Ziauddin University.
24 February 2024. Our PPIE members successfully completed their Informed Consent training guided by our PPIE coordinators. Since then, they have been meeting weekly to review the public-facing documents of the REMAP-CAP trial at Ziauddin University, to ensure clarity and relevance for our community. Collaborative efforts like these are vital for conducting effective research by emphasizing what is relevant within our context here in Karachi, to the wider research community. Our work highlights the importance of integrating patient and public partners in trial processes!
Event: Global Acute Care Colloquium in Toronto
6 October 2023. Dr. Timo Tolppa (PPIE group co-coordinator) shared the lessons learnt from setting up the PPIE group at Ziauddin with acute and critical care doctors, practitioners and researchers at an international meeting in Toronto, Canada. Attendees celebrated the success of the PPIE group in Pakistan and expressed a desire to hear more from the patients and the public in Karachi. The meeting also included talks from public representatives and other researchers engaging with patients across the world. There is clear interest internationally to include the patient voice meaningfully in the design and conduct of research, and the PPIE group in Pakistan is contributing to that aim.
Event: First PPIE Group Meeting
16 September 2023. Our PPIE group has held their first-ever meeting! This group of eight inspirational and dedicated members came together to start their exciting work on bringing the patient voice to clinical trials at Ziauddin University. During their first meeting, the group learned about the power of PPIE in clinical trials and discussed the ethical, moral and practical imperatives for meaningfully involving the public in research. The group also set ambitious aims and objectives to transform clinical trials in Pakistan to make them more patient-centered and societally beneficial.
Event: Lecture on Patient and Public Involvement and Engagement for Clinical Trials
1 June 2023. Dr. Timo Tolppa delivered an inspiring lecture to staff and faculty at Ziauddin University on “Patient and Public Involvement and Engagement for Clinical Trials” to introduce the crucial role patients and the public can play in shaping the future of clinical research. Dr. Tolppa shared his insights on the ways in which public engagement can lead to more inclusive, societally beneficial, and impactful clinical trials. Attendees discussed the opportunities, challenges, and future directions of involvement and engagement in Pakistan and at Ziauddin, and left the session empowered to begin the exciting work of fostering collaborative relationships with the community.
Event: Contract Signing Ceremony for the Establishment of the PPIE Group
1 June 2023. A contract signing ceremony was held at Ziauddin University to celebrate the historic establishment of the first-ever Patient & Public Involvement and Engagement Group in a Clinical Trials Unit in Pakistan. This ceremony was the culmination of months of hard work to seek funding, gain approvals, design the project, and recruit team members to allow this exciting group to be launched. We are extremely grateful to Mahidol Oxford Tropical Medicine Research Unit for providing us with funding and to Ziauddin University for enabling us to carry out this work. In attendance at the contract signing ceremony were Dr. Timo Tolppa (co-lead of the project from the University of British Columbia), Dr. Madiha Hashmi (Head of the Department of Critical Care Medicine at Ziauddin University), Dr. Zulfiqar Ali Umrani (Director of the Office of Research, Innovation, and Commercialization), Prof. Dr. Syed Irfan Hyder (Vice-Chancellor of Ziauddin University), and Prof. Dr. Abbas Zafar (Dean of the Faculty of Health Sciences at Ziauddin University).

Professor Dr. Nikhat Ahmed Siddiqui (Coordinator)
Professor Nikhat is a neuroscientist, biochemist, and former Dean of Research at Ziauddin University. Her research career spans over 38 years and crosses the entire research spectrum, from laboratory experiments to clinical studies on diseases such as diabetes, dementia, and blindness. She also meaningfully contributes to research ethics, training, policy development, and strategic planning. Beyond conducting scientific research, Prof. Nikhat is passionate about building capacity to enable high-quality and patient-centered research in Pakistan.

Dr. Arishay Hussaini (Coordinator)
Dr. Arishay is a clinical research associate in the Department of Critical Care Medicine at Ziauddin University. She has actively participated in the implementation and conduct of various research projects, including international randomised controlled trials, qualitative and observational studies. Her interests lie in exploring and improving the experiences of study participants. She aims to work with the local population in Karachi to set research priorities and make research findings relevant to Pakistan.

Dr. Timo Tolppa (Coordinator)
Dr. Timo is a Ph.D. candidate based at the University of British Columbia in Canada and has worked as a regional clinical trial coordinator for several international intensive care trials across South Asia, including Malaysia, Nepal, and Pakistan. He is working collaboratively with researchers at Ziauddin University on making clinical trials more relevant for low-resource settings. Dr. Timo is focused on patient engagement, qualitative research, and health equity, and has a personal interest in the use of film to promote health and justice.

Professor Dr. Madiha Hashmi (Senior Project Lead)
Dr. Hashmi is Professor & Chair of the Department of Critical Care Medicine at the Dr. Ziauddin Group of Hospitals and Ziauddin University. She is a physician researcher with extensive experience in all aspects of critical care research; she has acted as the principal investigator for international trials and set up a national intensive care registry in Pakistan (PRICE) to enable data driven quality improvement projects, observational studies, and clinical trials. Dr. Hashmi is focused on building critical care research capacity in Pakistan, which includes establishing and leading this patient and public involvement and engagement group.
We would love to hear from you!
Join us to learn more about research, help improve healthcare for our patients, and work collaboratively with us to make a difference in how research is conducted at Ziauddin University. Feel free to contact us via phone or email so we can keep you updated on our events, workshops, and opportunities to be involved. We also welcome any suggestions or feedback about our PPIE project.
Email. [email protected]
Phone. +92-3213660201

